Which of the Following Best Describes a Galvanic Cell
Aluminum Al oxidized zinc Zn reduced D. If concentration of Fe2is increased.
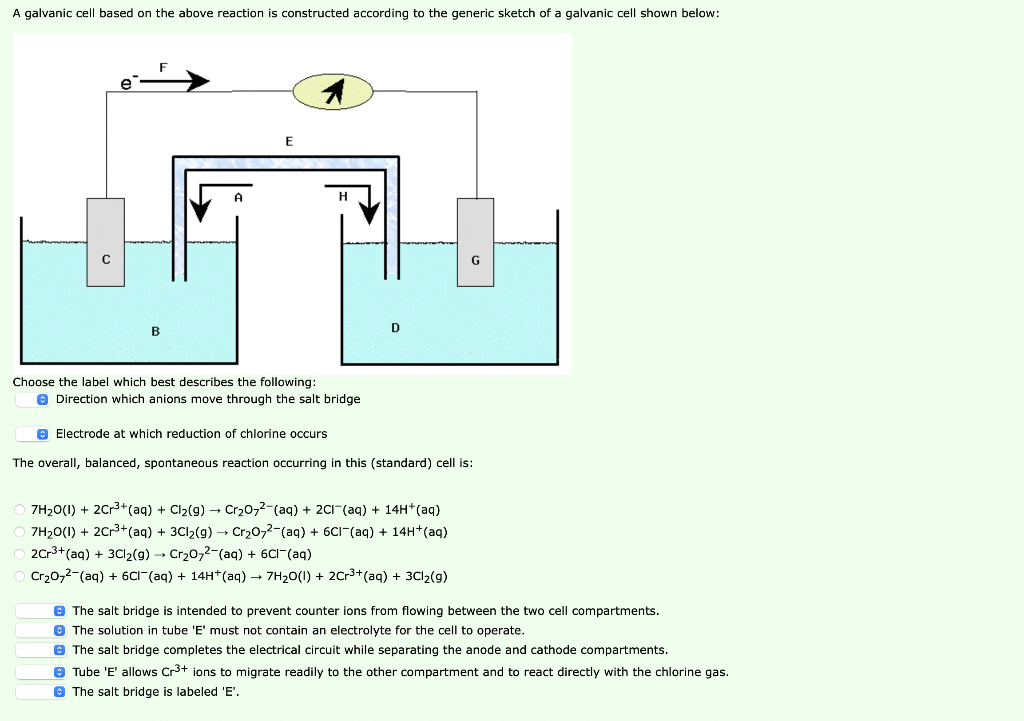
Solved A Galvanic Cell Based On The Above Reaction Is Chegg Com
Which of the following best describes the effect this substitution has on the initial reading on the voltmeter.
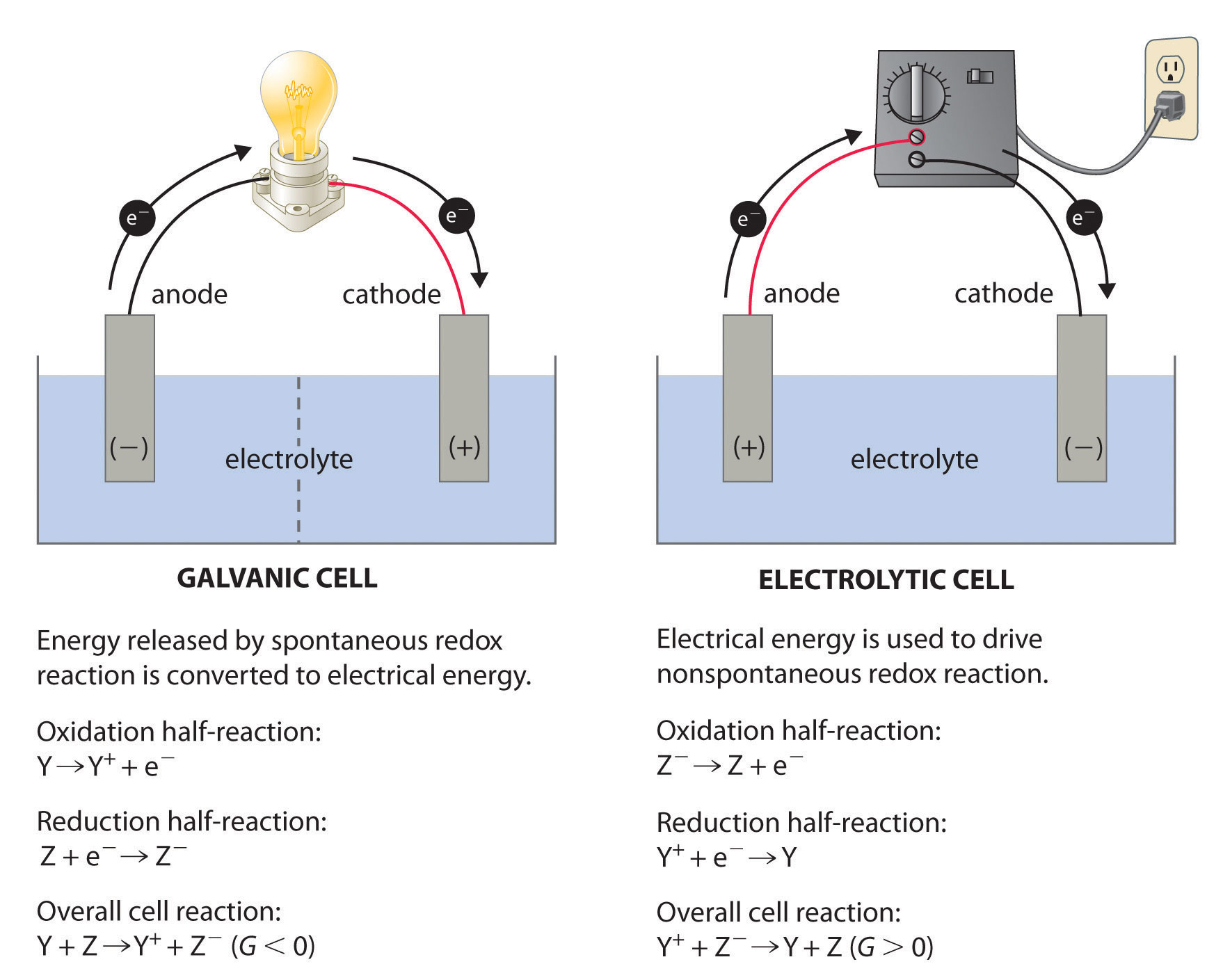
. The thode is negative. Describes the effect this substitution has on the initial reading on the voltmeter. Which of the following is an electrolytic cell.
Which of these statements best describes a galvanic cell. It involves the gain of electrons. ZnsZnCl2 aq Claq Cl2 gCs The reaction at anode in the electrochemical cell is.
Silver and chromium electrodes but substitutes a 050 M. B It provides a pathway for electrons to move between the half-cells. A fuel cell is a device that produces an electrical current as long as fuel and oxidizer are continuously added.
Which of the following statements best describes what would happen to the cell potential if the concentration of Cr2 is increased. A Galvanic cell is a battery and is made up of a conducting. Galvanic cell Voltaic cell is an electrochemical cell that makes use of chemical reactions to generate electrical energy.
Electrode A is the anode since anions are present in its half-cell and the current flows from cathode to anode. B The cell potential would become more positive. Table of Contents Principle of Galvanic Voltaic Cell.
Abattery that requires energy to charge it B. The cell potential measures the amount of current that is flowing in a galvanic cell. 2in ELECTROLYTIC CELL current is.
Reduction occurs at the anode. A battery containing an acid and a base C. A second galvanic cell is made from the same two metals and the measured cell potential is 325 V.
It involves the loss of electrons. See a chart showing the default reduction potential. Solution of silver nitrate for the 10 M solution of silver.
Which one of the following statements best describes the role of the salt bridge in a galvanic cell. A fuel cell is a galvanic cell in which the two half-cells are the same except for the concentration of the solutes. The galvanic cell shown above generates a cell potential of 317V when operated under standard conditions.
A standard galvanic cell consists of Cr3- Cr2 and CD2- Cd half-cell compartments connected by a salt bridge. The student constructs another galvanic cell still using silver and chromium electrodes but substitutes a 050 M solution of silver nitrate for the 10 M solution of silver nitrate in the silver half-cell. It occurs when a non-metal ion becomes an element.
Which statement best describes a reduction reaction. It requires an oxidizing agent. Choose all those that apply In the external circuit the electrons flow from the CD2- Compartment D at the Cr3- Cr2 Bay.
Silver Ag oxidized gold Au reduced B. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow of electrons through the conductor whereas in an electrolytic cell the redox reactions are influenced by an external source of current. Which of the following could be the reason for the second cell having a greater cell potential.
Which of the following best describes this electrochemical cell. In GALVANIC CELLelectric current is produced as a result of spontaneous redox reactionDaniel cell and fuel cell r galvanic or voltaic cell. Local system administrators b.
An electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy is known as a galvanic cell or a voltaic cell. Information security requires participation and support from which one of the the following groups. Lithium Li oxidized zinc Zn reduced C.
A Galvanic cell is simply defined as an electrochemical cell that uses the movement of electrons in a reduction-oxidation reaction to produce electrical energy for use. For an electrochemical reaction occuring in a galvanic cell. A battery containing a nonspontaneous reaction.
Electrode C is the cathode since cations are present in its half-cell. Option B Solution In any galvanic cell oxidation occurs at anode and reduction occurs at cathode. A battery containing a spontaneous redox reaction D.
Which of the following statements is correct. Oxidation occurs at the anode. That is option C.
Reduction occurs at the anode. The student constructs another galvanic cell still using. The galvanic cell shown above generates a cell potential of 317 V when operated under standard conditions.
Which of the following factors affect the rate of corrosion. The option that best describes a galvanic cell is that a battery containing a spontaneous redox reaction. Construct a labelled diagram for the following cell.
Which of these statements best describes a galvanic cell. It occurs at the anode of a galvanic cell. IronII Fe oxidized aluminum AI reduced 2 See answers Advertisement Advertisement brandyputnam brandyputnam Answer.
A second galvanic cell is made from the same two metals and the measured cell potential is. Know more differences between galvanic cells and electrolytic cells by visting us. Consider the following galvanic cell reaction at 25 oC 4 Cr2aq O2g 4 H3Oaq 4 Cr3aq 6 H2Ol.
It allows electrons to flow from one cell to another. A 50 kJ of heat is transferred to the surroundings. Nitrate in the silver half-cell.
Expert Answer 100 5 ratings. The three minimum requirements for a. A The cell potential would become less positive.
Which of the following best. A fuel cell is a primary battery that uses an alkaline electrolyte. Which of the following best describes a galvanic cell.
Among N a H g S P t and graphite which can be used as electrodes in electrolytic cells. Energy is required from an external source. A It allows positive charges to accumulate in one half-cell and negative charges to accumulate in the other.
Which of the following best describes the flow of heat when 10 mol of XY2 decomposes. 2Feaq3 Zns Znaq2 2Feaq2. Which statement best describes the role of the salt bridge in a galvanic cell.

Redox Wikipedia The Free Encyclopedia Galvanic Cell Electrochemistry Chemistry

Galvanic Cell An Overview Sciencedirect Topics

Galvanic Vs Electrolytic Cell Electrochemistry Ap Chemistry Chemistry
Galvanic Cells Chemistry For Majors

Lesson Worksheet Galvanic Cells Nagwa
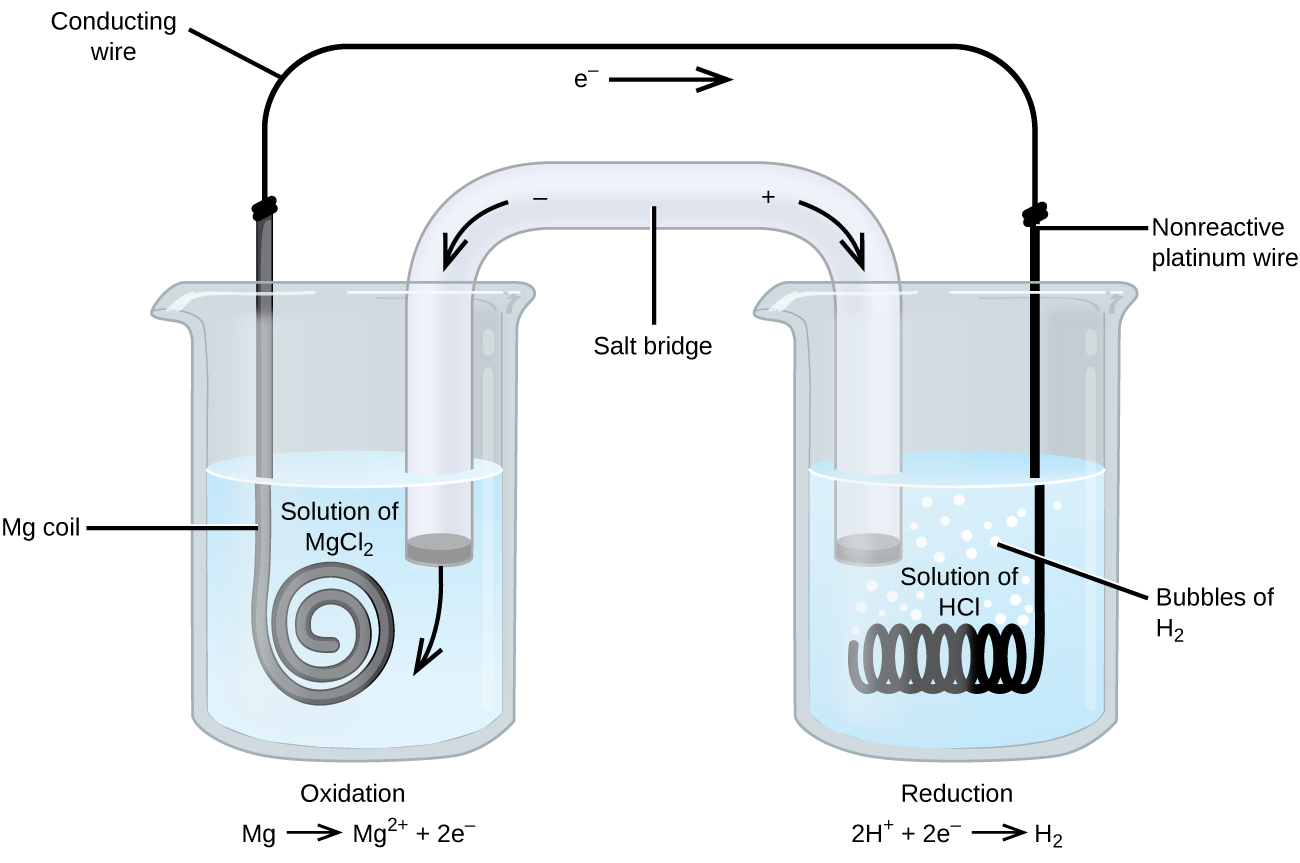

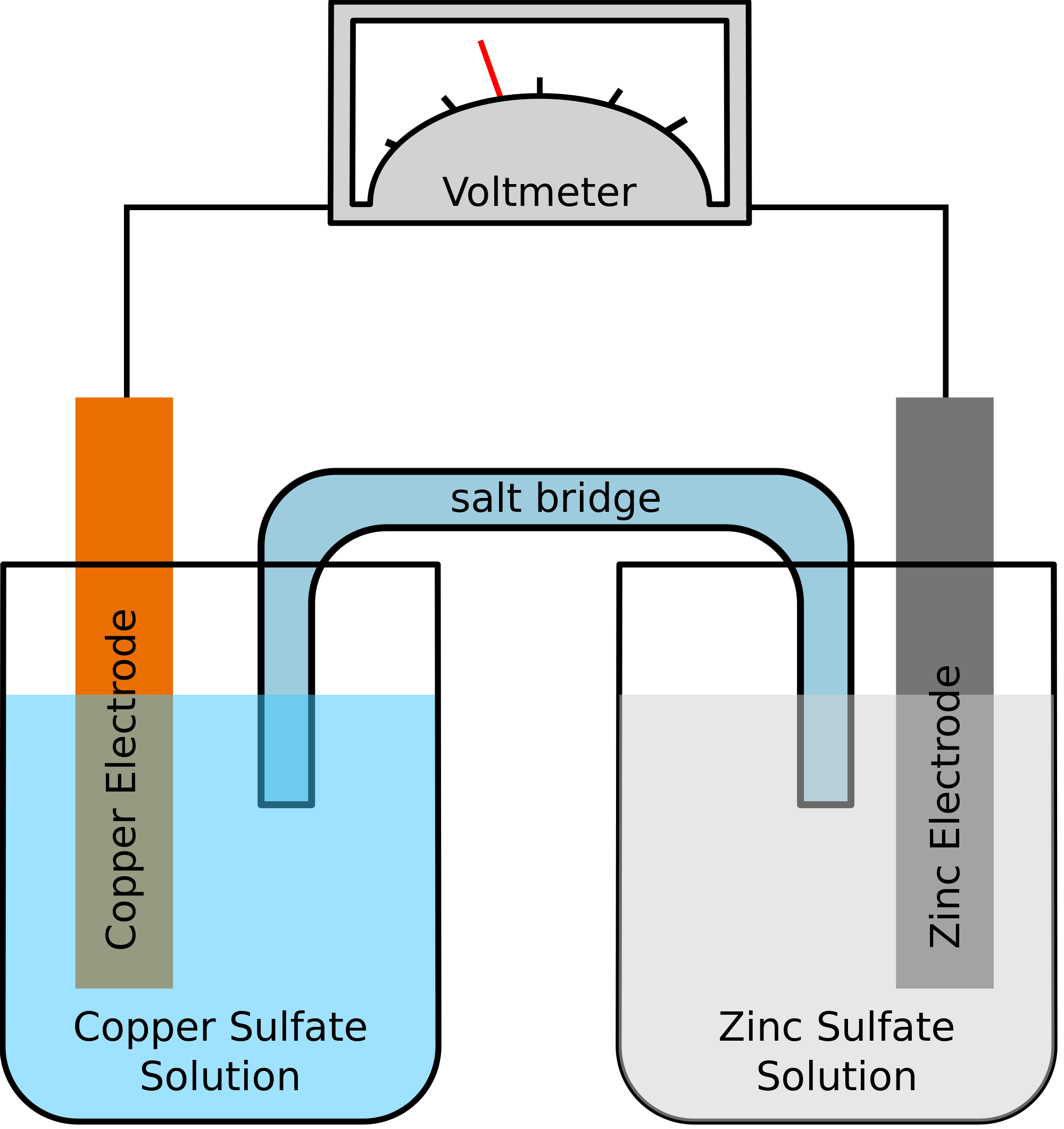
No comments for "Which of the Following Best Describes a Galvanic Cell"
Post a Comment